
BrF5 Molecular Geometry Science Education and Tutorials
An explanation of the molecular geometry for the BrF5 (Bromine pentafluoride) including a description of the BrF5 bond angles. The electron geometry for the.

DOWNLOAD How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride .Mp4 & MP3, 3gp
Step 1: Find the total number of valence electrons one molecule of BrF5 has: It is 42 as 7 is coming from each of the fluorine and bromine atoms. Step 2: Find how many more valence electrons are required by one molecule of BrF5: It is 6 as one valence electron is required by each participating atom.

BrF5 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Techiescientist
BrF5 lewis structure has a Bromine atom (Br) at the center which is surrounded by five Fluorine atoms (F). There are 5 single bonds between the Bromine atom (Br) and each Fluorine atom (F). There are 3 lone pairs on all the Fluorine atoms (F) and 1 lone pair on the Bromine atom (Br).

DOWNLOAD How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride .Mp4 & MP3, 3gp
The BrF5 or the bromine pentafluoride has a bond angle of 90°. Therefore, the angle formed between the central atoms and the other ones has an angle of 90° between them. As for the total number of 90° bond angles in the bromine pentafluoride, the compound consists of 5 bond pairs of atoms and one lone pair. Also, it has to be kept in mind.

DOWNLOAD How To Draw The Lewis Dot Structure For Brf5 Bromine Pentafluoride .Mp4 & MP3, 3gp
The BrF5 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the BrF5 molecule. The geometry of the BrF5 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the BrF5 geometrical shape in which the electrons have from one another.
Reidesignsforyou Lewis Dot Structure For Brf5 Free Nude Porn Photos
In the BrF 5 Lewis structure, there are five single bonds around the bromine atom, with five fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the bromine atom has one lone pair. Contents Steps #1 First draw a rough sketch #2 Mark lone pairs on the atoms #3 Calculate and mark formal charges on the atoms, if required

Hey is this structure of BrF5 is correct Chemistry Chemical Bonding and Molecular Structure
BrF5 Geometry and Hybridization. Br is the central atom, so we can draw a preliminary skeletal structure: There are 5×7 + 7 = 42 electrons, out of which, 10 are used to make 5 covalent bonds. The remaining 30 are divided between the five fluorine atoms, each taking 6 electrons as 3 lone pairs, and Br takes the last pair of electrons: There are.

Among the molecules SO2, SF4, ClF3,BrF5andXeF4. Which of the following shapes does not describe
There is an easy three-step process for determining the geometry of molecules with one central atom. Step 1: Determine the Lewis structure of the molecule. For BrF 5, it is as shown below: For a full-explanation of how to figure out the Lewis structure, please go to Lewis Structure of BrF 5. However, here is what it looks like.

BrF5 Lewis Structure How to Draw the Lewis Dot Structure for BrF5 YouTube
Step-by-step video of how to get from the formula BrF5 to its Lewis structure and geometry. AboutPressCopyrightContact usCreatorsAdvertiseDevelopersTermsPrivacyPolicy & SafetyHow YouTube.

Best Overview Is BrF5 Polar or Nonpolar Science Education and Tutorials
The Lewis structure of BrF5 contains five single bonds, with bromine in the center, and five fluorines on either side. There are three lone pairs on each fluorine atom, and one lone pair on the bromine atom. How to Draw the Lewis Dot Structure for BrF5: Bromine pentafluoride Watch on Contents Steps #1 Draw skeleton #2 Show chemical bond
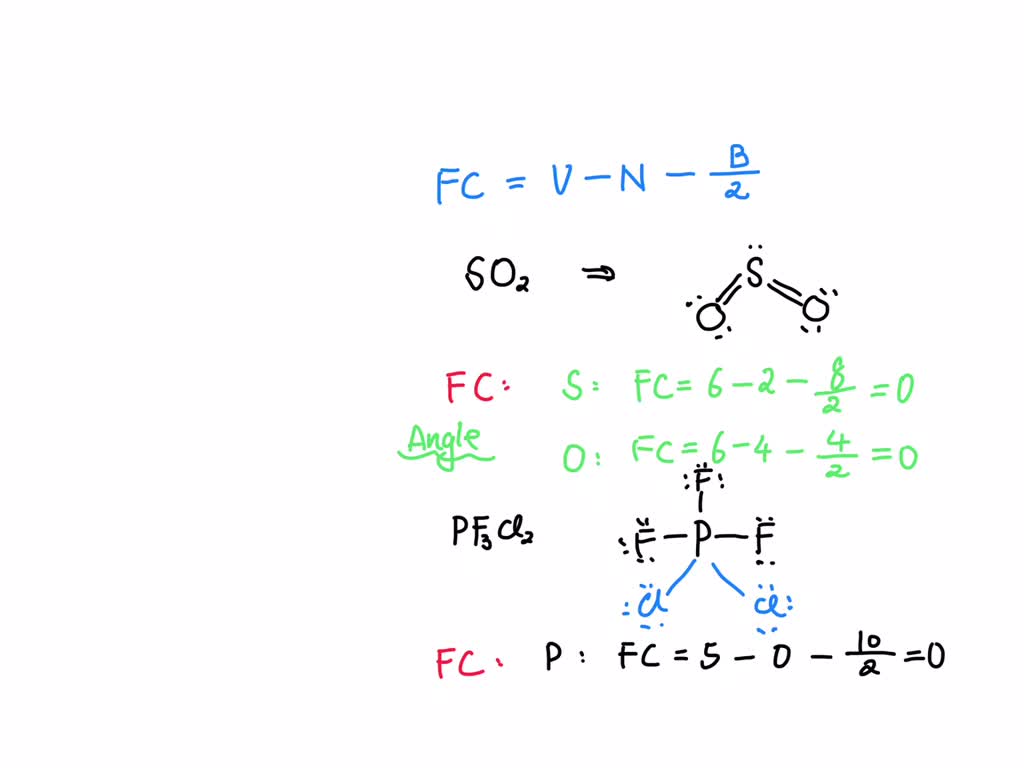
SOLVED Geometry So2,PF3CL2,BrF5,CLF3.Draw the lewis structure calculate the formal charges and
The BrF5 Lewis structure is a way to represent the arrangement of atoms and electrons in the molecule. It involves drawing a diagram that shows the valence electrons of the bromine and fluorine atoms as dots and lines that represent covalent bonds between them.

Formal charge on bromine atom of BrF5 molecule = (7 2(10/2)) =0
Bromine pentafluoride is one of the most reactive halogen fluorides, probably outperformed in its reactivity only by ClF 3. 1 - 5 BrF 5 was discovered by Ruff and Menzel in 1931 and described as a colorless liquid that freezes at 211.85 K (−61.30 °C) and boils at 313.65 K (+40.50 °C). 6 BrF 5 is the highest known binary fluoride of bromine.

Explain the shapes of brf5. Brainly.in
The Lewis structure of BrF5 tells that there are seven valence electrons in bromine, and seven valence electrons in fluorine. Steps to draw the Lewis Structure of BrF5 The steps have to be followed to draw the Lewis structure of BrF5 Step 1: At first we have to calculate the valence electrons in bromine pentafluoride molecule.

The first step is to put seven valence electrons around the bromine atom as given in the figure.
1. The central atom, sulfur, contributes six valence electrons, and each fluorine atom has seven valence electrons, so the Lewis electron structure is. With an expanded valence, that this species is an exception to the octet rule. 2. There are six electron groups around the central atom, each a bonding pair.
What is the Hybridization of Bromine Pentafluoride
336 94K views 10 years ago A step-by-step explanation of how to draw the BrF5 Lewis Dot Structure (Bromine pentafluoride). For the BrF5 structure use the periodic table to find the total.

BrF5 Molecular Geometry & Bond Angles (Bromine Pentafluoride) YouTube
Lewis structure of BrF5 contains five single bonds between the Bromine (Br) atom and each Fluorine (F) atom. The Bromine atom (Br) is at the center and it is surrounded by 5 Fluorine atoms (F). The Bromine atom has 1 lone pair while all the five Fluorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.